Facility
PET
In our center, we are conducting new imaging research using positron emission tomography (PET) with originally developed ligands and analysis methods for elucidating the pathogenesis of various diseases. .
1, Equipments
Positron emission tomography (Fig. 1)
The PET scanner for humans and small animals is used in our center for various purposes.
The high resolution (1.6 mm) and high detection sensitivity of the PET scanner for small animals are used to visualize the physiological processes and to develop pharmaceuticals and biological research by the pharmacokinetic analysis with the radiopharmaceuticals.
The human PET-CT system has been development and practice the visualization method of specific membrane proteins behavior with the original ligands and the analyzed methods developed at our center.
Fig. 1: Human (left) and small animal (right) PET scanners
Labeled compound synthesis system (Fig. 2)
This facility is equipped with a small negative ion accelerated cyclotron that produces positron-emitting radionuclides (11C, 18F, 15O, etc.) and synthesizes various labeled compounds.
Each the equipment for synthesis of 15O-labeled water ([15O]H2O), oxygen, carbon monoxide, etc. in the PET/CT room and those of 11C and 18F in the shielding device called a Hot Cell is installed.
We have synthesized an Aquaporin agent ([11C]TGN020), two kinds of glucose analogues ([18F]3FDG and [18F]2FDG) and a β-amyloid detection agent ([18F]flutemetamol) inside the Hot Cell.

Fig. 2: Cyclotron (left), 15O (center), 11C, 18F (right) labeled compound synthesis system
2, Research Results
Aquaporin PET (Fig. 3, 4)
We succeeded in visualizing aquaporin distribution in vivo for the first time in the world using an aquaporin-targeting agent [11C]TGN020, which was originally developed at our center. (Ref. 1,2)
With this imaging technique, we reported that the appearance of aquaporin increases with the malignancy of astrocytoma (Ref. 3, Fig. 5) and that aquaporin is abundantly expressed in the skull unlike other bones, e.g. the ribs and humerus (Ref. 4, Fig. 6).
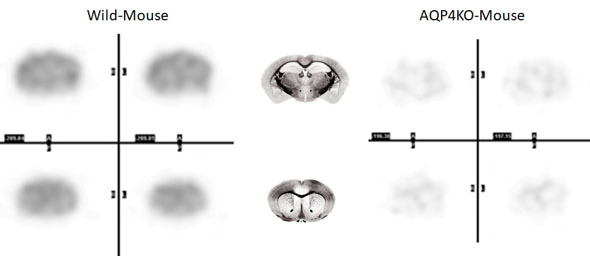
Fig. 3; [11C]TGN-020 PET images (wild-type vs. AQP4 knockout mouse brain images)
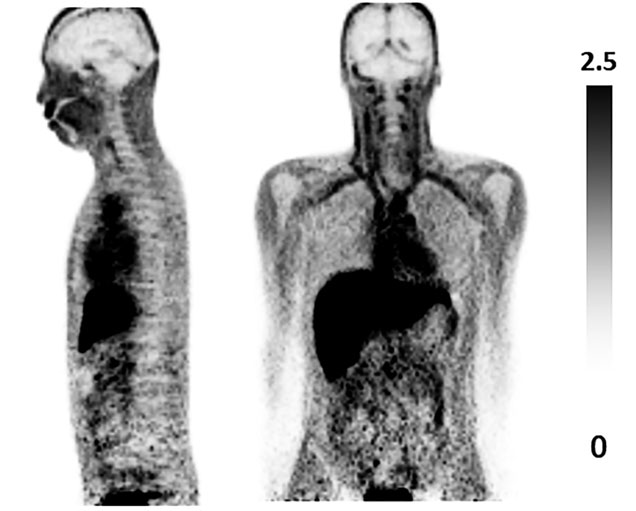
Fig. 4; [11C]-TGN-020 PET image (human whole body images)
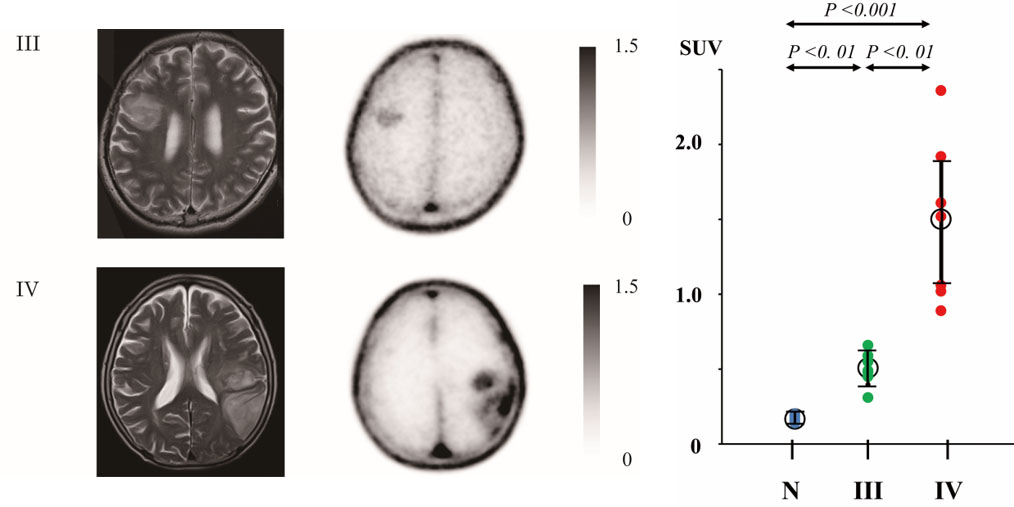
Fig. 5; MRI and Aquaporin PET images of glioma (left). The uptake of [11C]TGN-020 significantly increases with the malignancy (right).
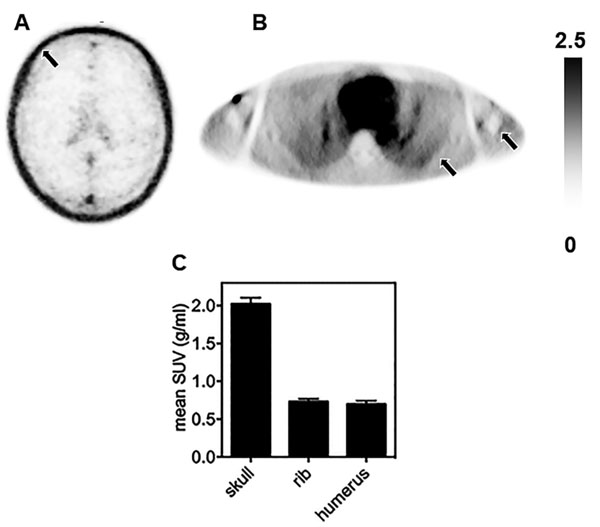
Fig. 6; Aquaporin in the skull (A) is clearly more abundant than in the ribs, humerus (B) and other bones. (C)
Assessment of glymphatic system function
We analyzed water flow in the brain using 15O-labeled water ([15O]H2O) to assess the glymphatic system function, which is known as the lymphatic system of the brain. Using this assessment method, we reported that glymphatic system function had been impaired in Alzheimer’s patients (Ref. 5, Fig. 7). Detailed investigation of the relationship between the accumulation of β-amyloid, which is observed early in the development of Alzheimer’s disease, and the function of the glymphatic system can lead to the elucidation of the pathogenesis and the development of treatments.
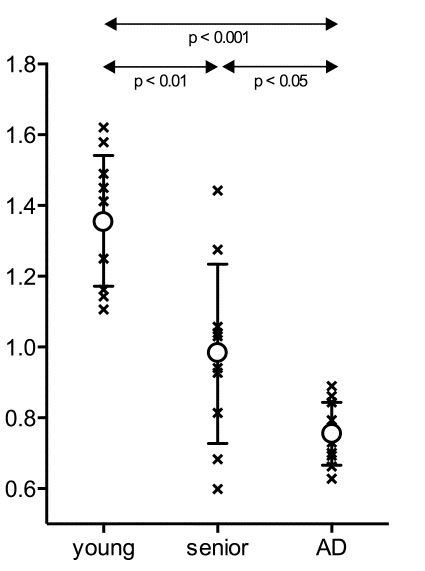
Fig. 7; In Alzheimer’s patients (AD), the water flow in the brain is significantly reduced compared to healthy individuals of the same age (senior).
Reference
- 1. Development of a Novel Ligand, [11C]TGN-020, for Aquaporin 4 Positron Emission Tomography Imaging. Nakamura Y, Suzuki Y, Tsujita M, Huber VJ, Yamada K, Nakada T. ACS Chem Neurosci. 2011 Oct 19;2(10):568-71.
- 2. Aquaporin-4 positron emission tomography imaging of the human brain: first report. Suzuki Y, Nakamura Y, Yamada K, Huber VJ, Tsujita M, Nakada T. J Neuroimaging. 2013 Apr;23(2):219-23.
- 3. Aquaporin PET differentiates between grade III and IV human astrocytoma. Suzuki Y, Nakamura Y, Yamada K, Kurabe S, Okamoto K, Aoki H, Kitaura H, Kakita A, Fujii Y, Huber VJ, Igarashi H, Kwee IL, Nakada T, Neurosurgery. 2018;82(6):842-6
- 4. Skull diploë is rich in aquaporin-4. Suzuki Y, Kitaura H, Nakamura Y, Kakita A, Huber VJ, Capozzoli N, Kwee IL, Nakada T. Heliyon. 2020 (vol6) Jan, E03259
- 5. Reduction of water influx into CSF in Alzheimer’s disease, supporting the β-amyloid clearance hypothesis. Suzuki Y, Nakamura Y, Yamada K, Igarashi H, Kasuga K, Yokoyama Y, Ikeuchi T, Nishizawa M, Kwee IL, Nakada T. PLOS ONE. 2015 May 6;10(5): e0123708.

